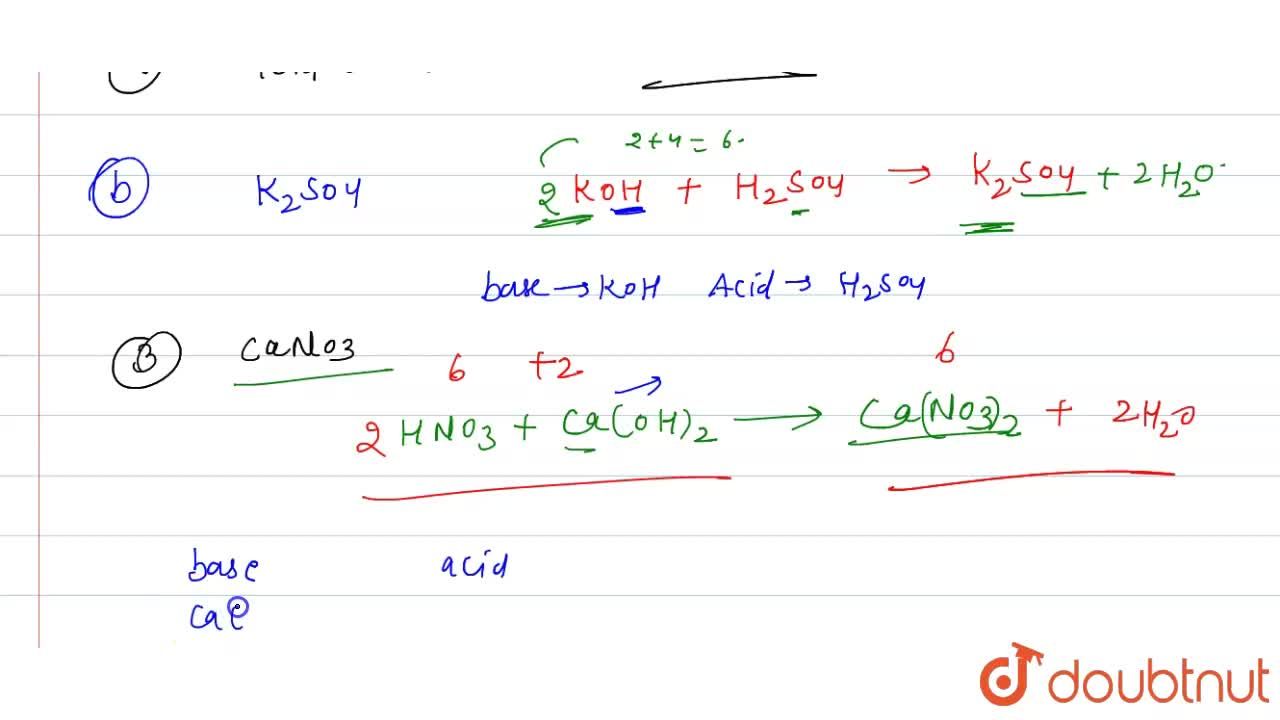
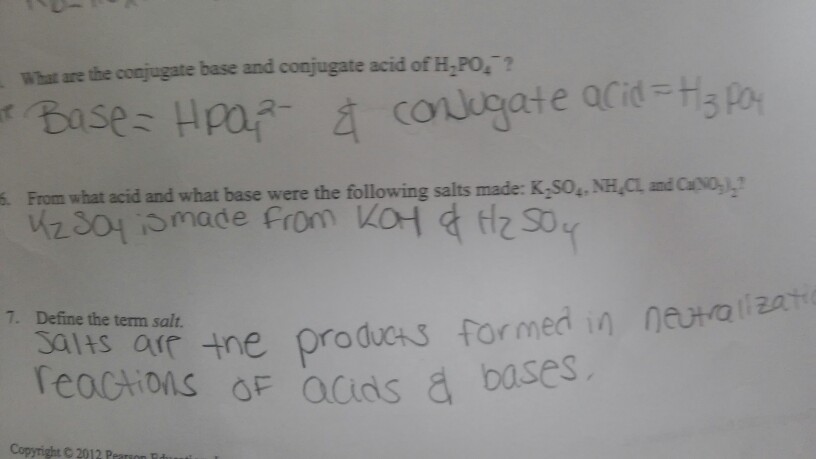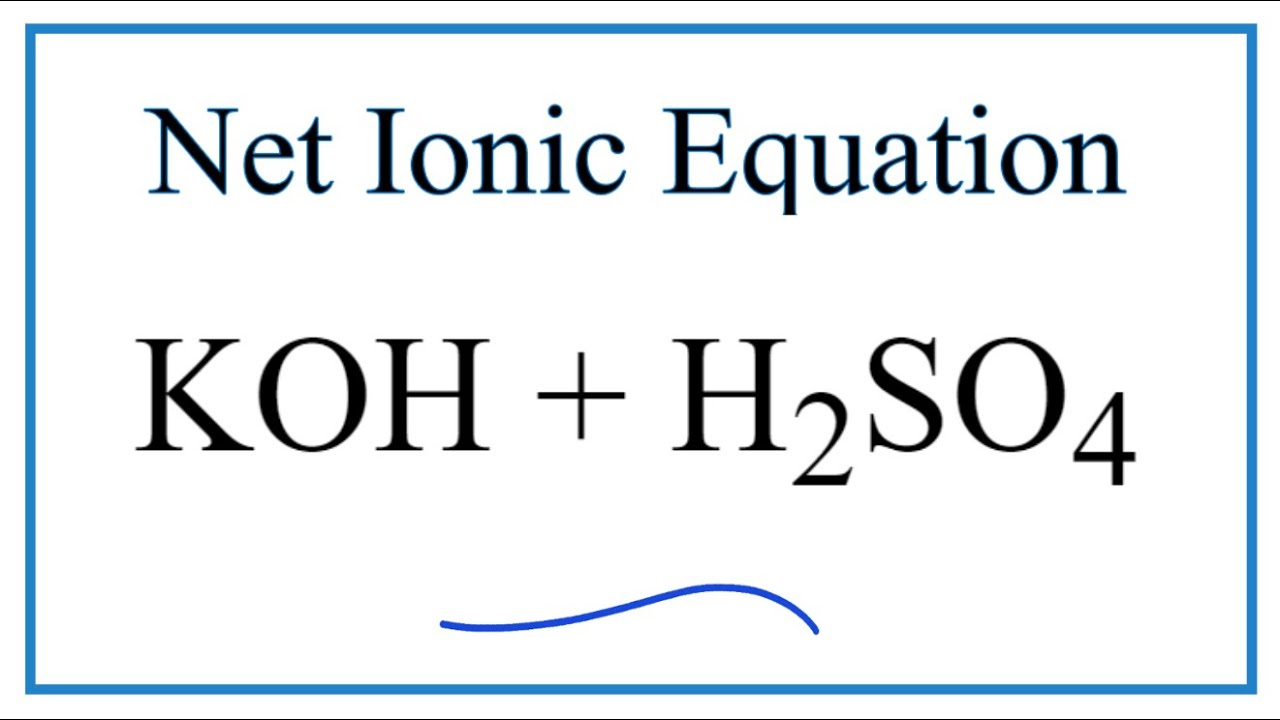Match the column I with column II and mark the appropriate choice.Column IColumn II(A) K2SO4.Al2(SO4)3.24H2O(aq) (i)Bronsted base(B)Salt of weak acid and strong base(ii)Neutral(C) H2PO4^- (iii)Basic(D)Solution of pH 6.5 at high temperature(iv)Acidic
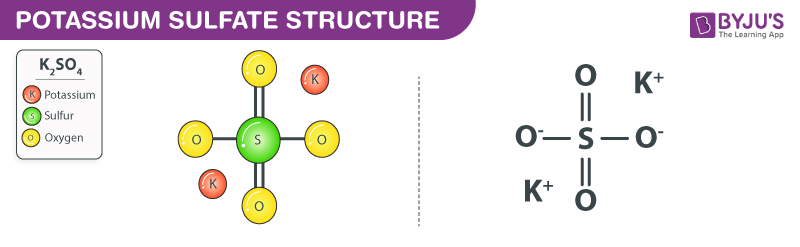
Potassium sulfate (K2SO4) - Structure, Molecular mass, Properties, Uses and FAQs of Potassium sulfate (K2SO4)

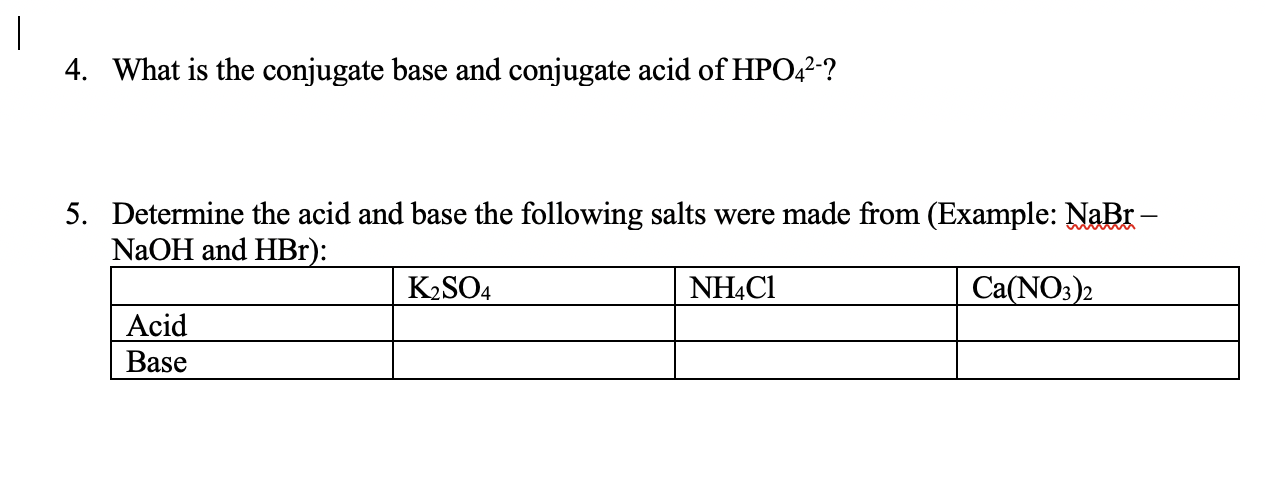
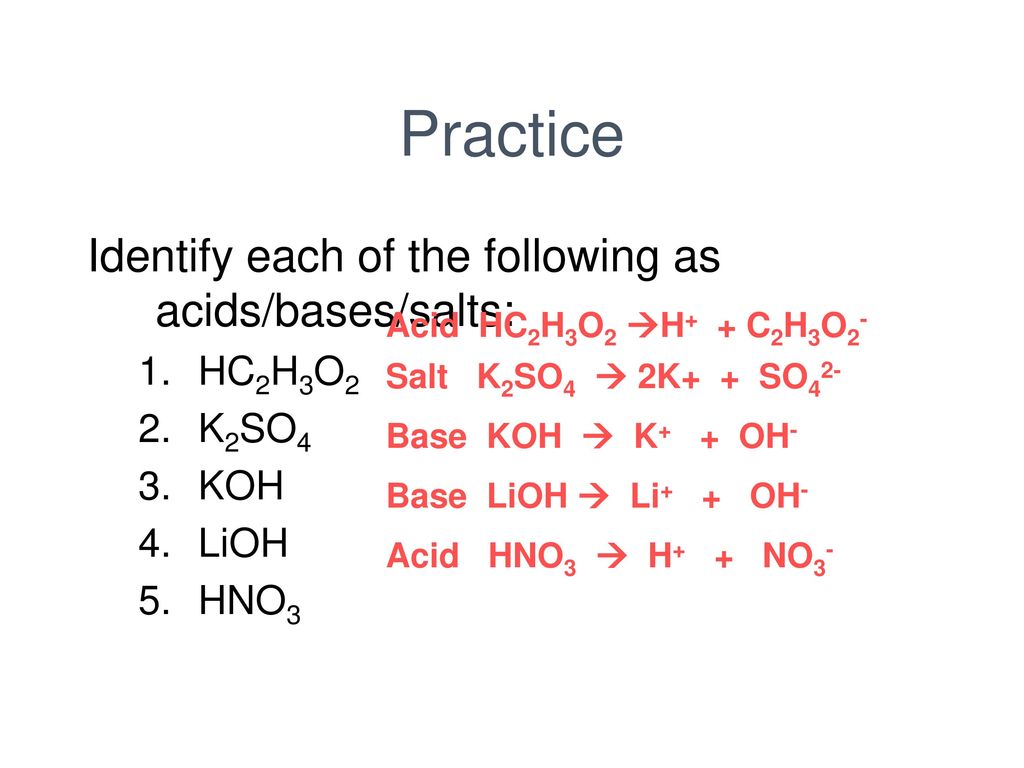
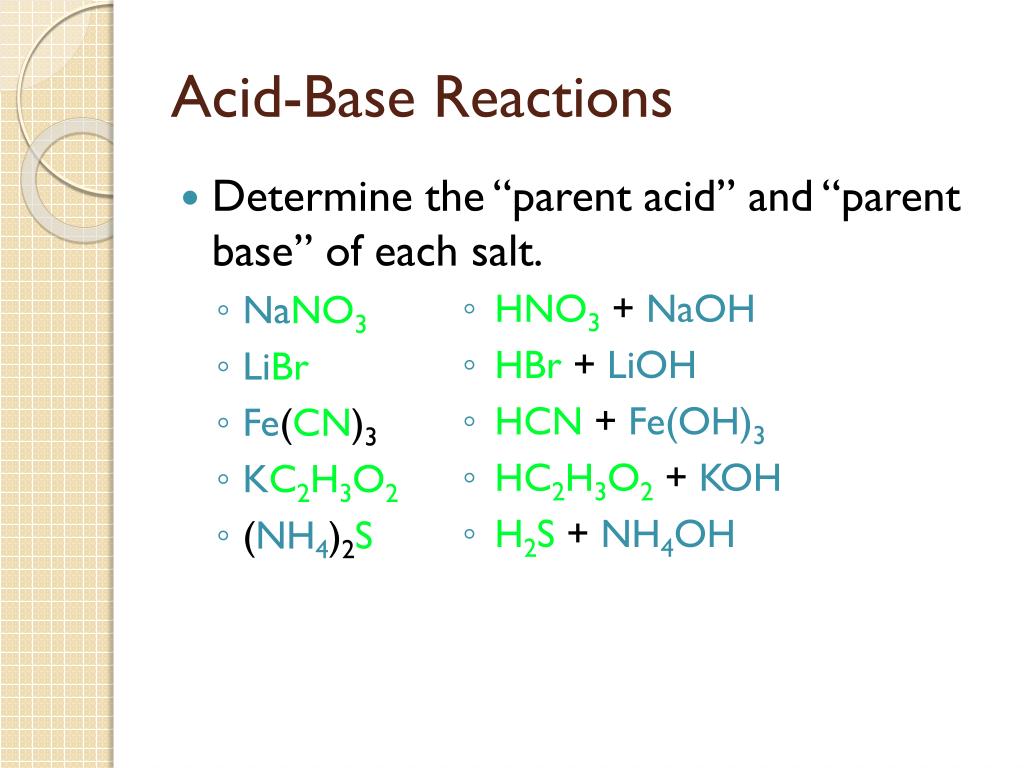
Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl