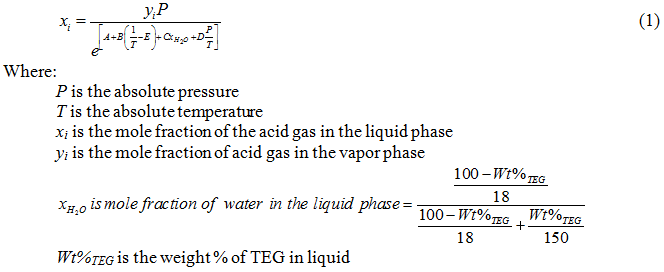
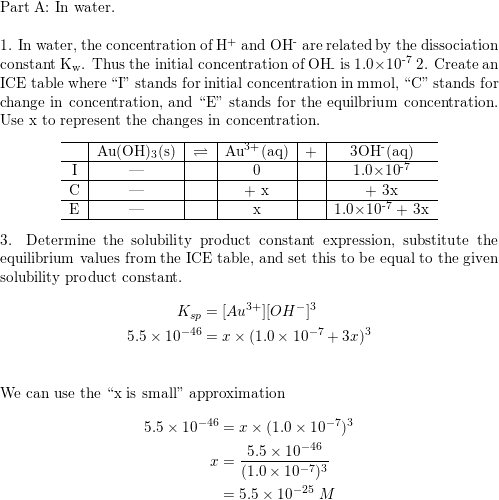
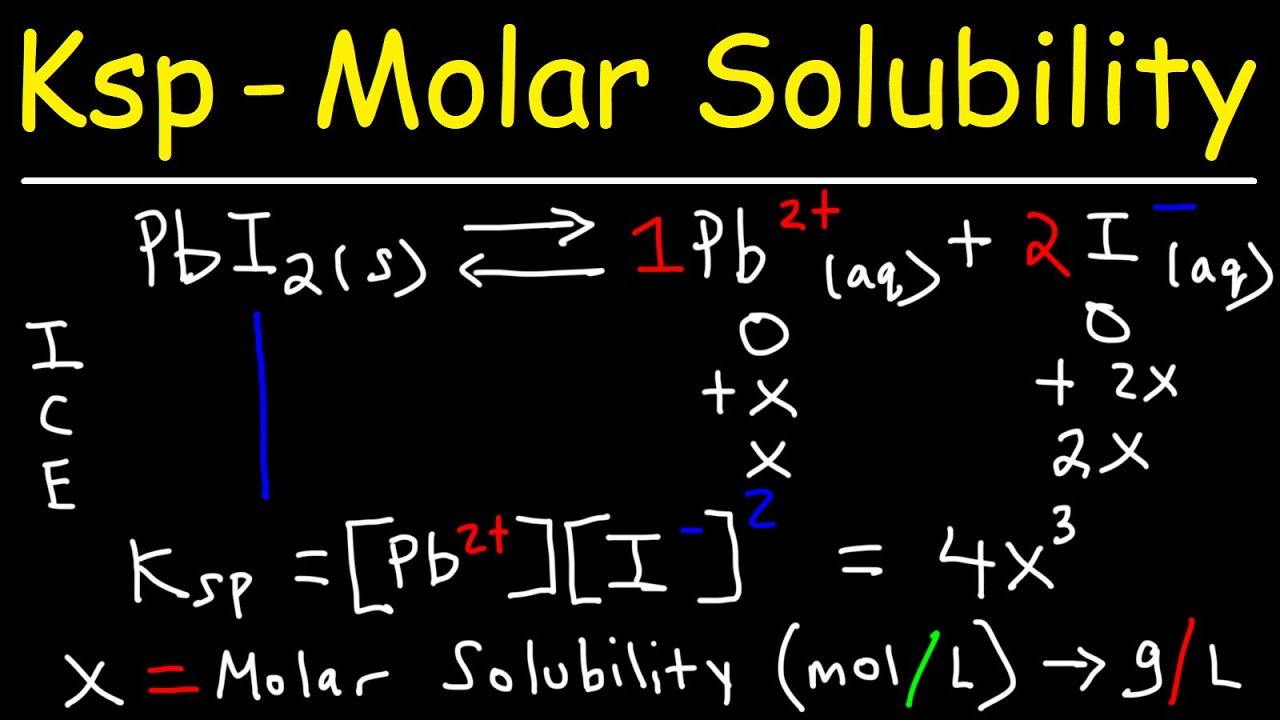
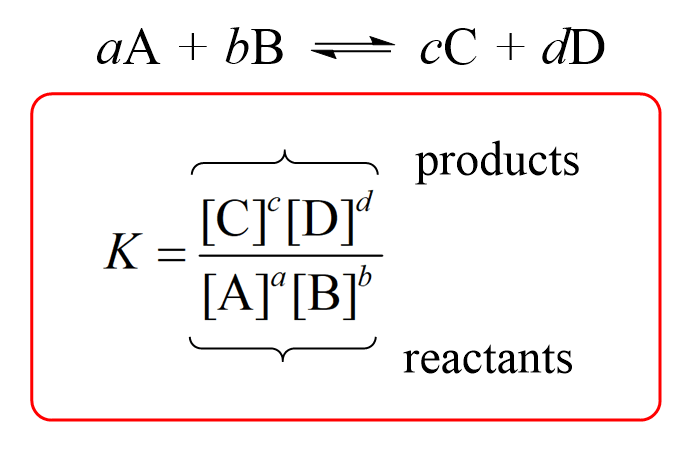
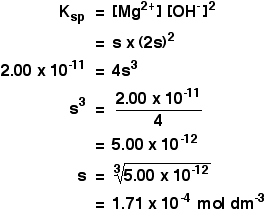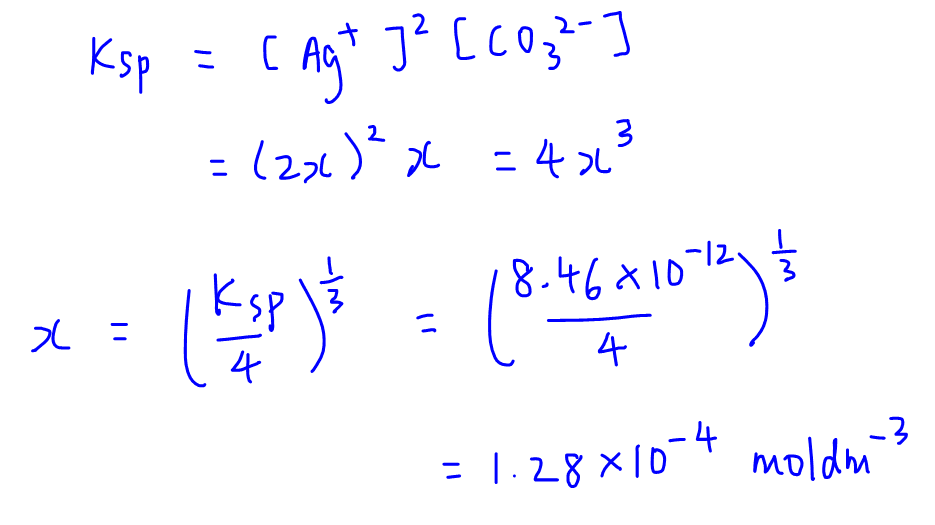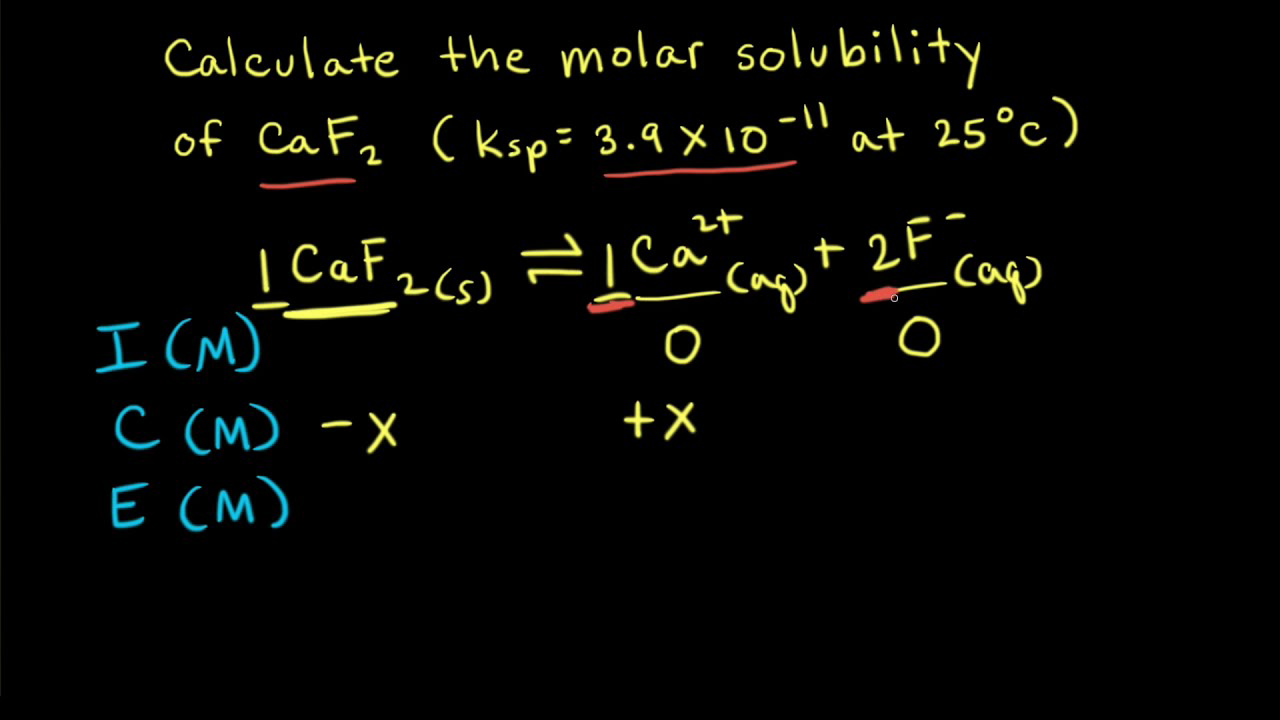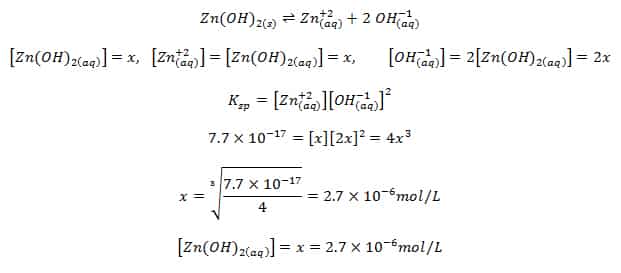What is the solubility of Pb(OH)2 in a buffer solution of pH8 if its solubility in water is 6.7*10^-6? - Quora
![Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download](https://images.slideplayer.com/27/9060864/slides/slide_3.jpg)
Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download
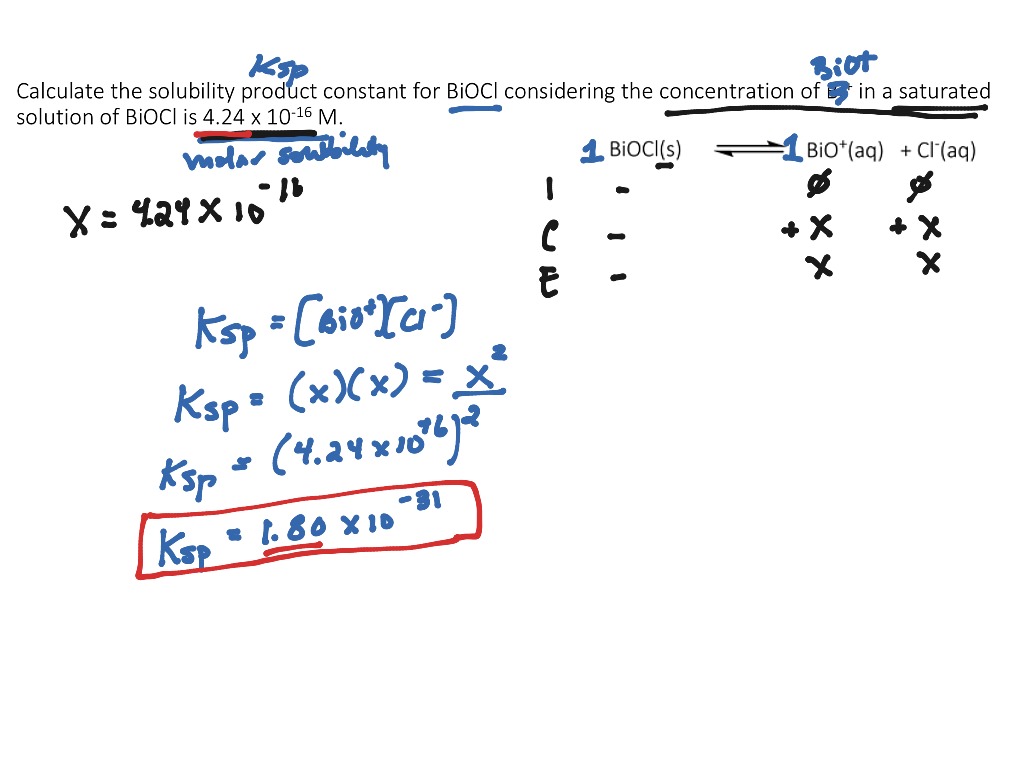
Calculating the Solubility Product Constant (Ksp) for an Insoluble Salt | Science, Chemistry | ShowMe
![SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH) SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)](https://cdn.numerade.com/ask_images/7d7ca9e3ac22444fbaeef36ffd903405.jpg)
SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)