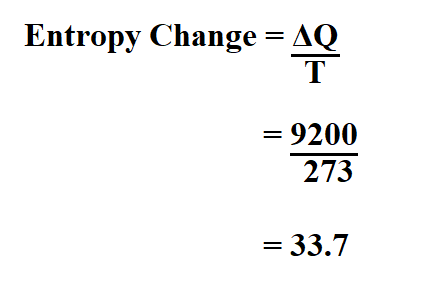Calculate the entropy change in surroundings when 1.00 mol of H2O (l) is formed under standard conditions at 298k, Given ,H^o = - 286kJ mol^-1

How to Calculate the Entropy Change for a Chemical or Physical Process Based on Absolute Entropies | Chemistry | Study.com

Calculate the entropy change in surrounding when 1.00 mol of H2O(l) is formed under standard condition fH^ = - 286 KJ mol^-1 .
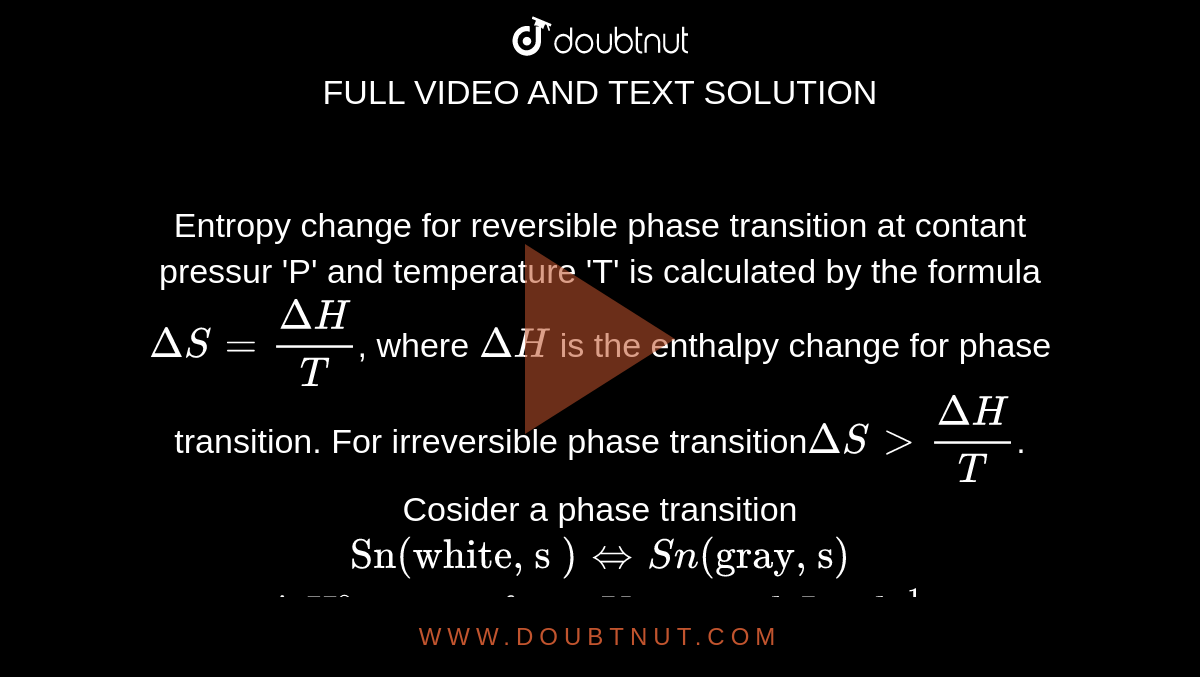
Entropy change for reversible phase transition at contant pressur 'P' and temperature 'T' is calculated by the formula DeltaS=(DeltaH)/(T), where DeltaH is the enthalpy change for phase transition. For irreversible phase transitionDeltaS
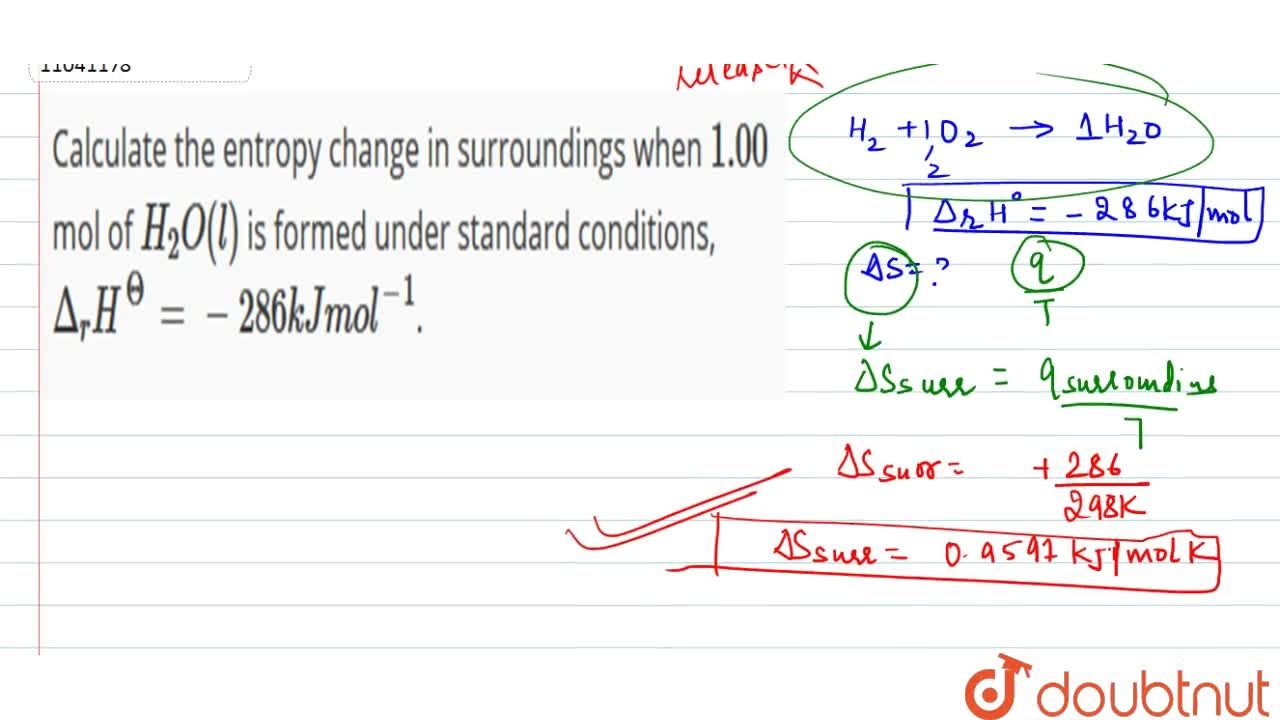
Calculate the entropy change in surroundings when 1.00 mol of H(2)O(l) is formed under standard conditions, Delta(r )H^(Θ) = -286 kJ mol^(-1).

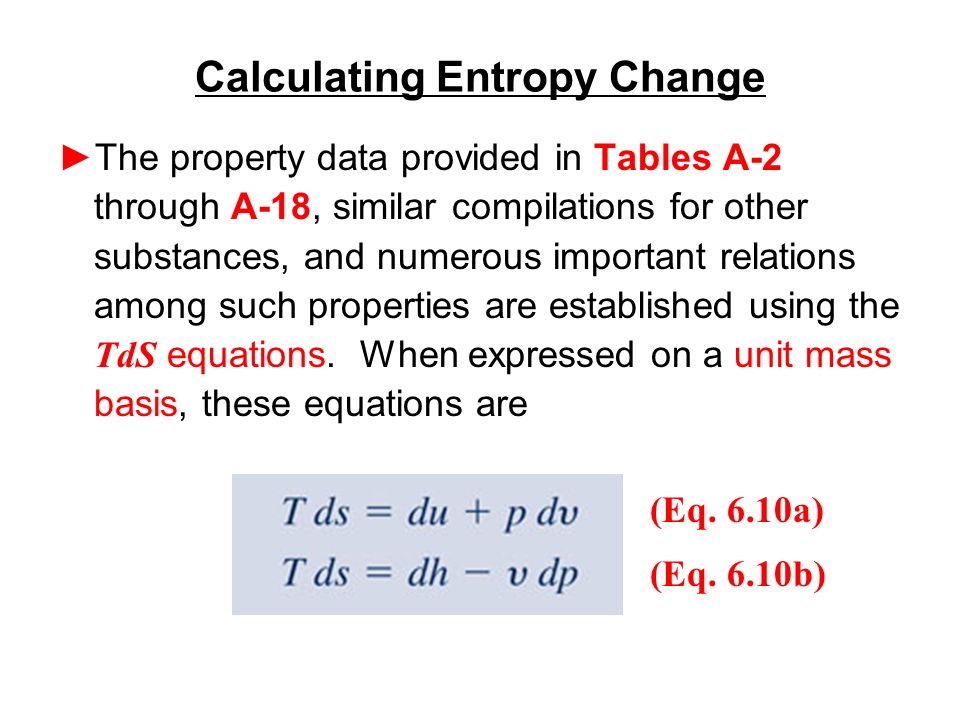
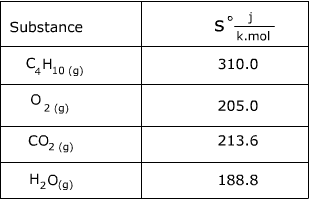

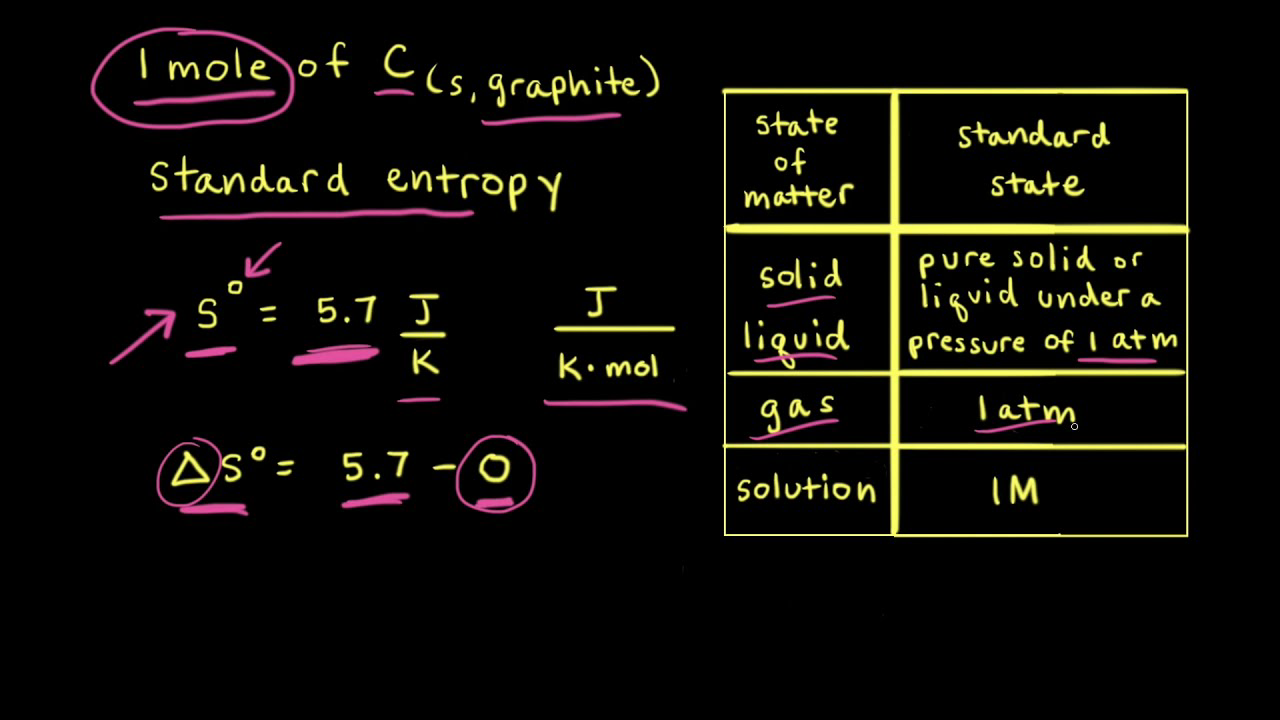

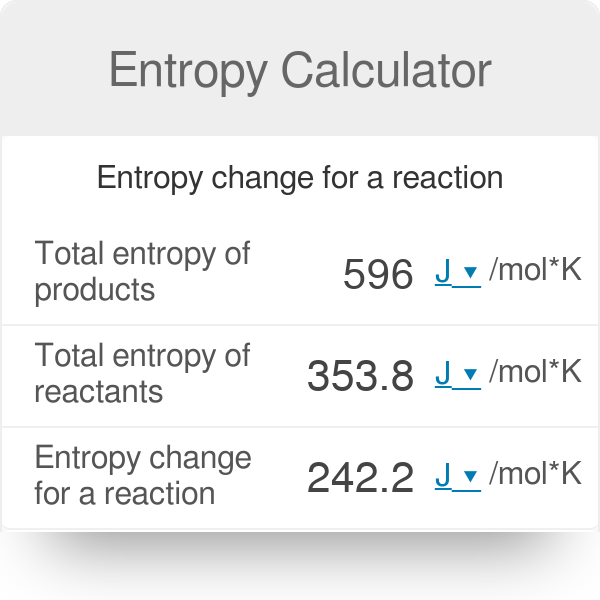
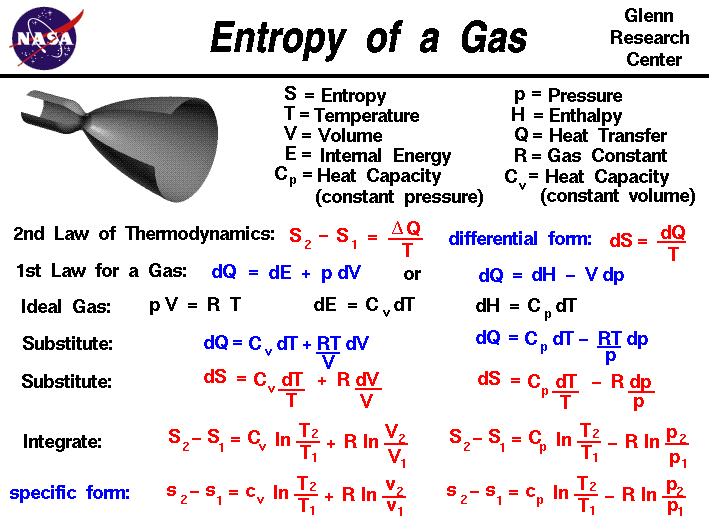

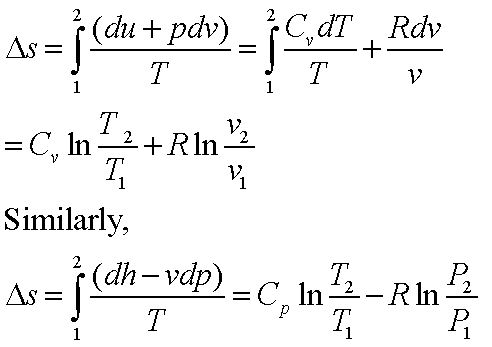
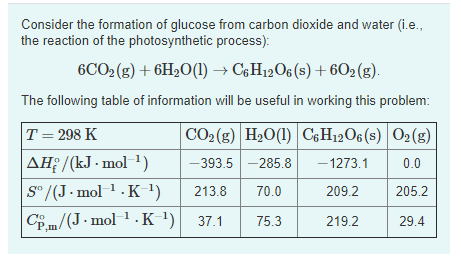



![15.2/R1.4 Calculate the standard entropy change for a reaction [HL IB Chemistry] - YouTube 15.2/R1.4 Calculate the standard entropy change for a reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/IwRy4iYVQLI/maxresdefault.jpg)