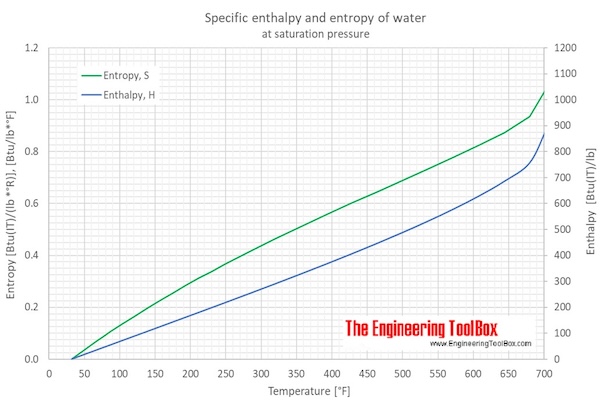
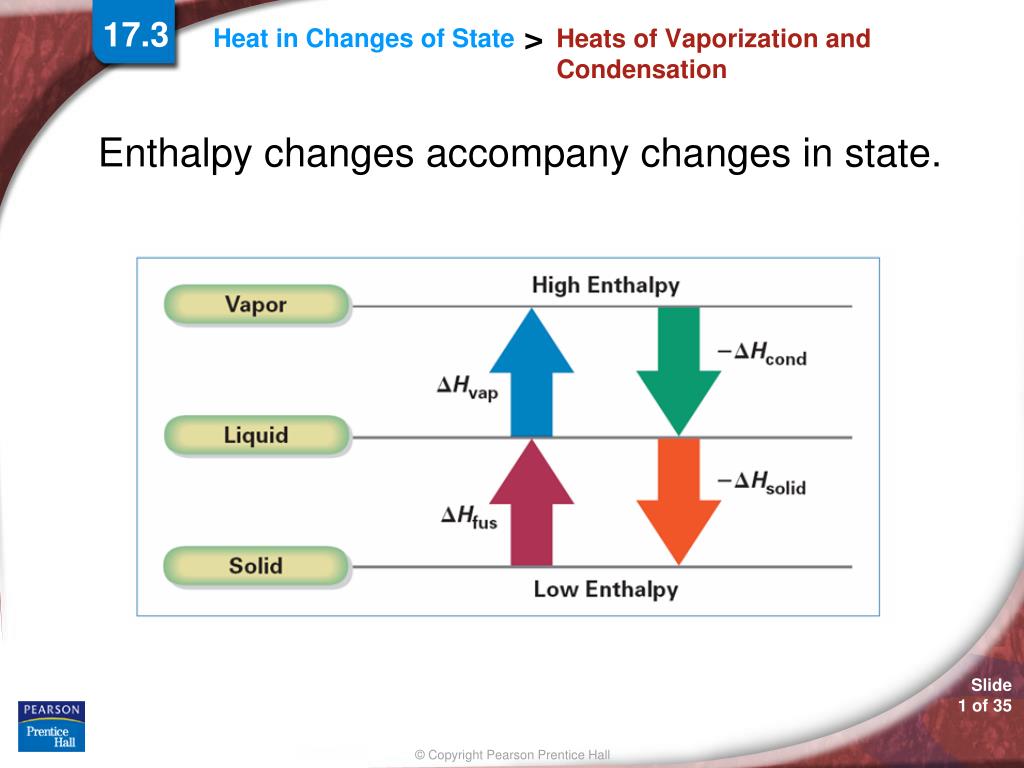
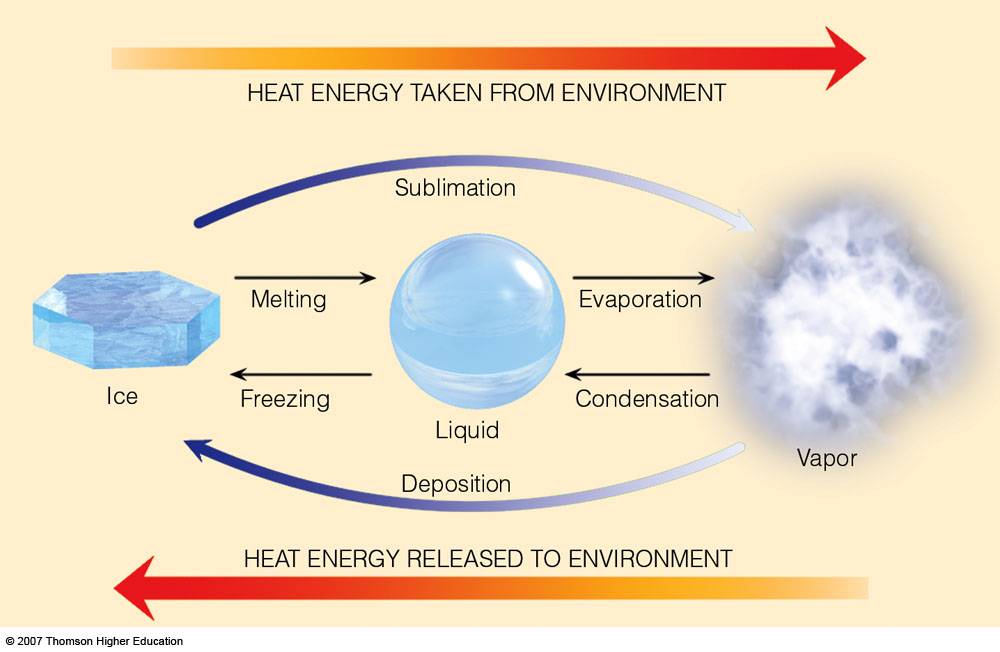
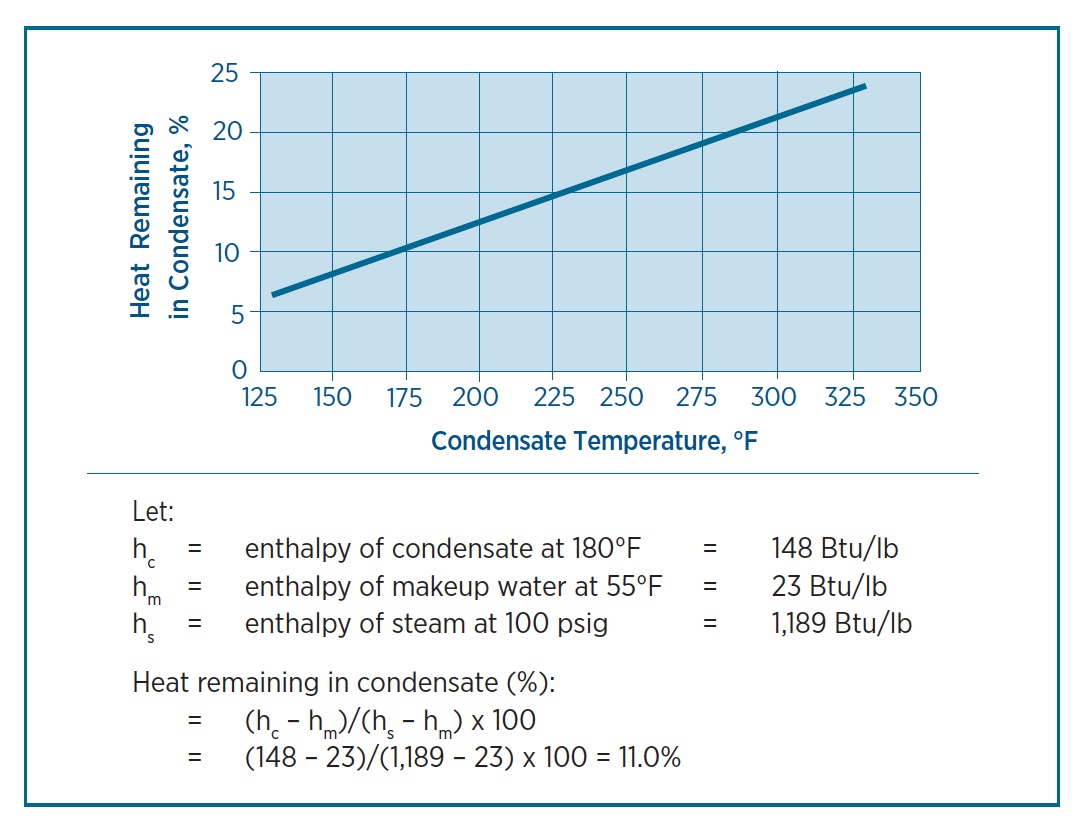
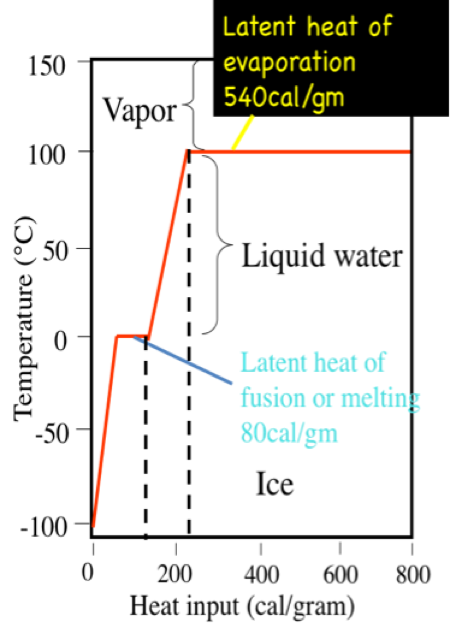
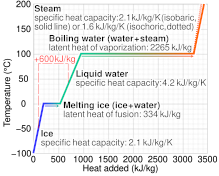
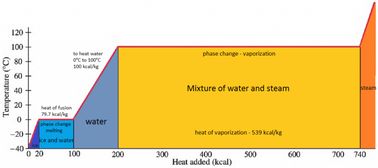
enthalpy - What is heat of vaporization? How can it be used at temperature as low as 25 °C? - Chemistry Stack Exchange
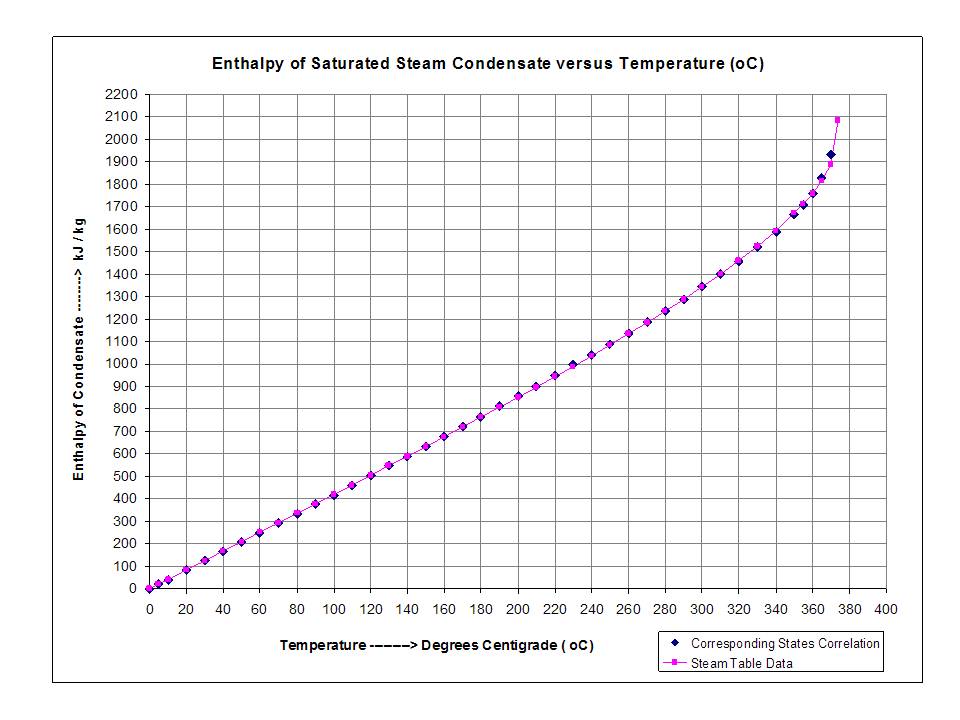
Calculation of Enthalpy and Density of Condensate ( saturated liquid Water) by corresponding states correlation | Chem-Eng-Musings

SOLVED: 16 The enthalpy of evaporation f water is 40.1 kJ/mol, what is the enthalpy of condensation of 36.04 g of water? (a) 2.35 KJ (b) -2.35 KJ (c) -80.2 KJ (d)
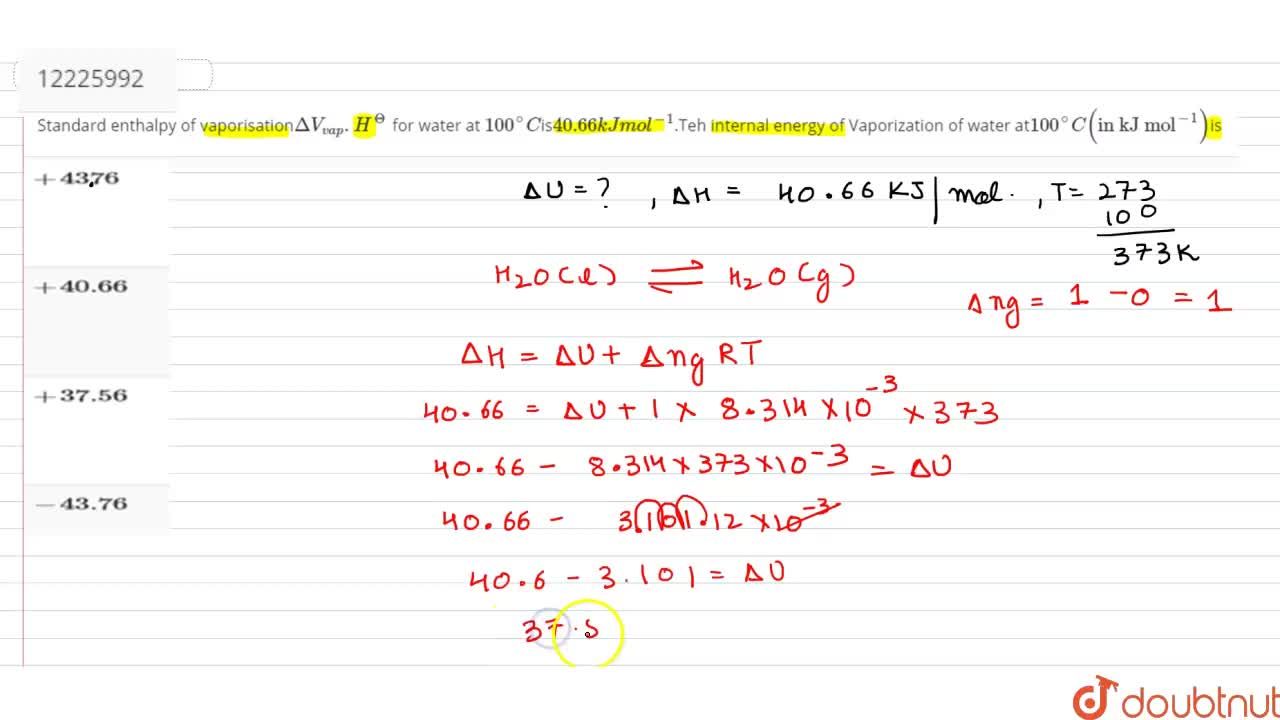
Standard enthalpy of vaporisationDeltaV(vap).H^(Theta) for water at 100^(@)Cis40.66kJmol^(-1).Teh internal energy of Vaporization of water at 100^(@)C("in kJ mol"^(-1))is