
pH of when 50mL of 0.10 M ammonia solution is treated with 50 mL of 0.05 M HCI solution :- ` - YouTube

In the titration of 50.0 mL of 0.10 M ammonia (K_b = 1.8 times 10^{-5}), calculate the pH: 1 ) Before titration begins 2 ) After addition of 20.0 mL of 0.10 M hydrochloric acid 3 ) After addition | Homework.Study.com

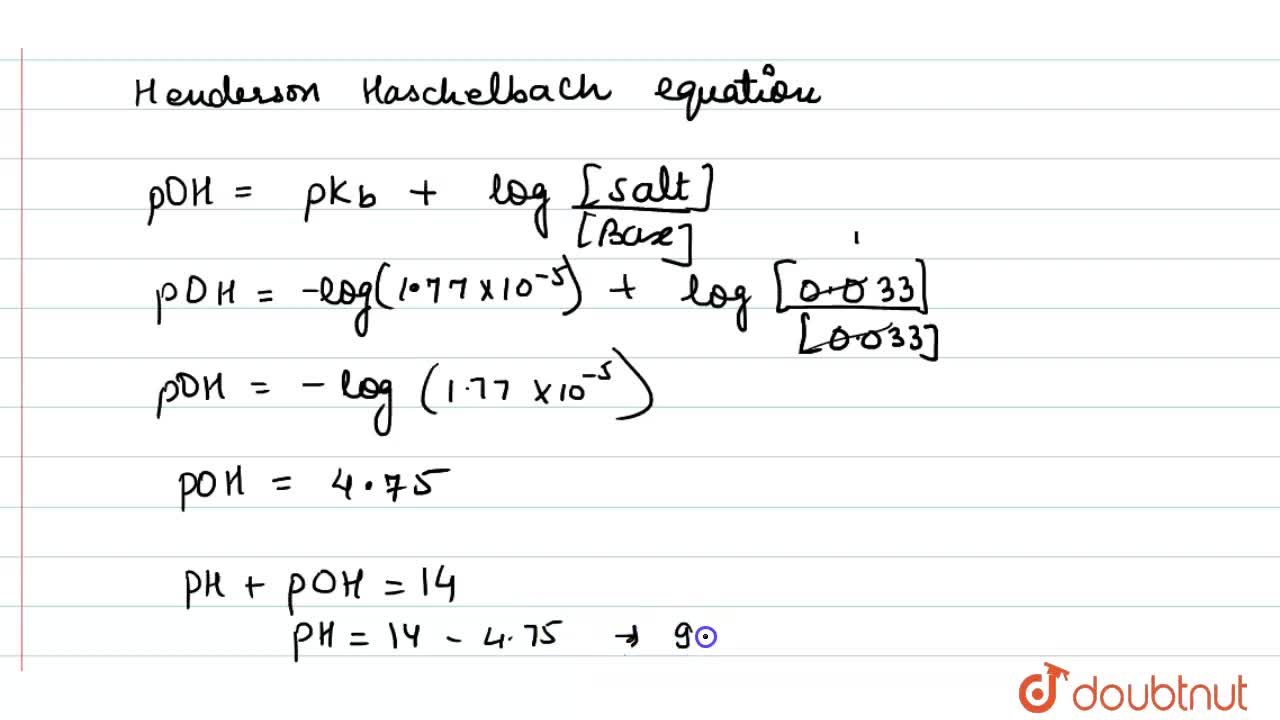
How can we calculate the pH of the solution in which 0.2 M NH4Cl and 0.1 M NH3 are present and the pKb of ammonia solution is 4.75 .

Calculate the pH of 0.10M ammonia solution. Calcualte the pH after 50.0mL of this solution is treated with 25.0mL of 0.10M HCl. The dissociation constant of ammonia, K(b)=1.77xx10^(-5).
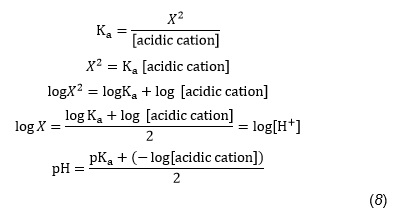
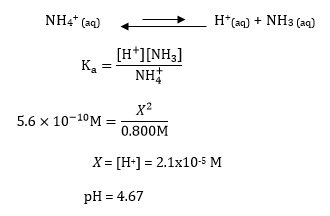









![Solved in a Calculate the approximate [OH ] and [NH 0.15 M | Chegg.com Solved in a Calculate the approximate [OH ] and [NH 0.15 M | Chegg.com](https://media.cheggcdn.com/media%2F987%2F987f3c98-c3a8-47ea-a9fe-f5b98a6a126e%2Fimage)



