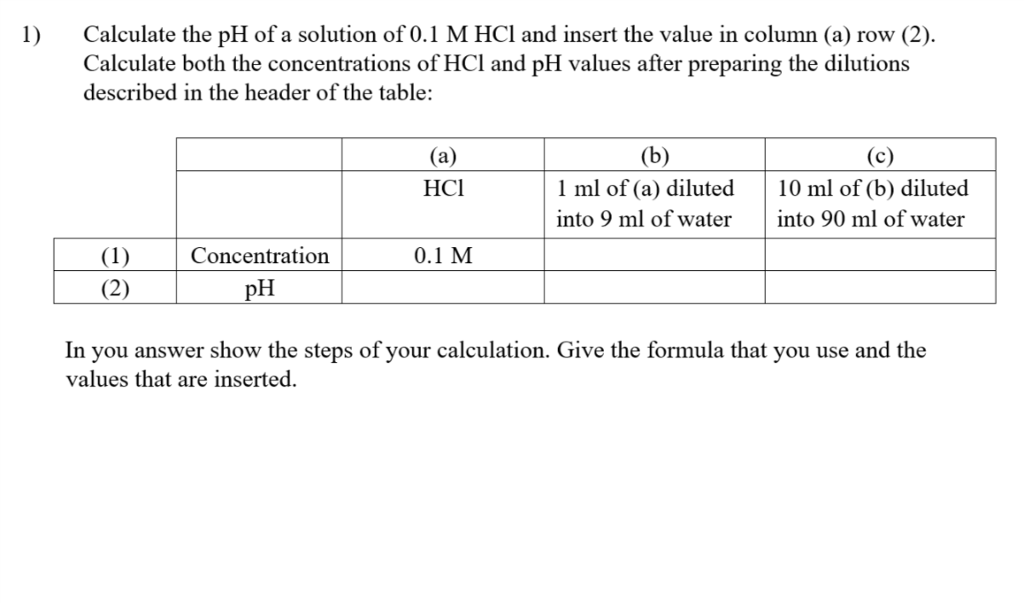
What is the pH of a solution in which 25.0 mL of the 0.1 M NaOH is added to 25 mL of 0.08 M HCl and final solution is siluted to 500 mL ?
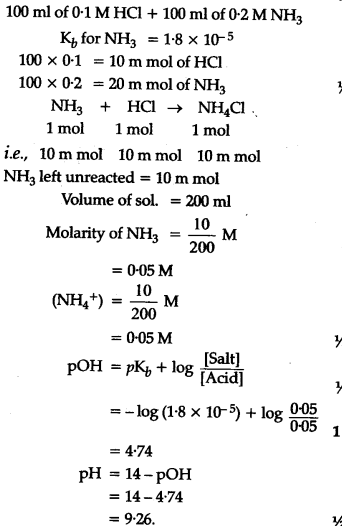
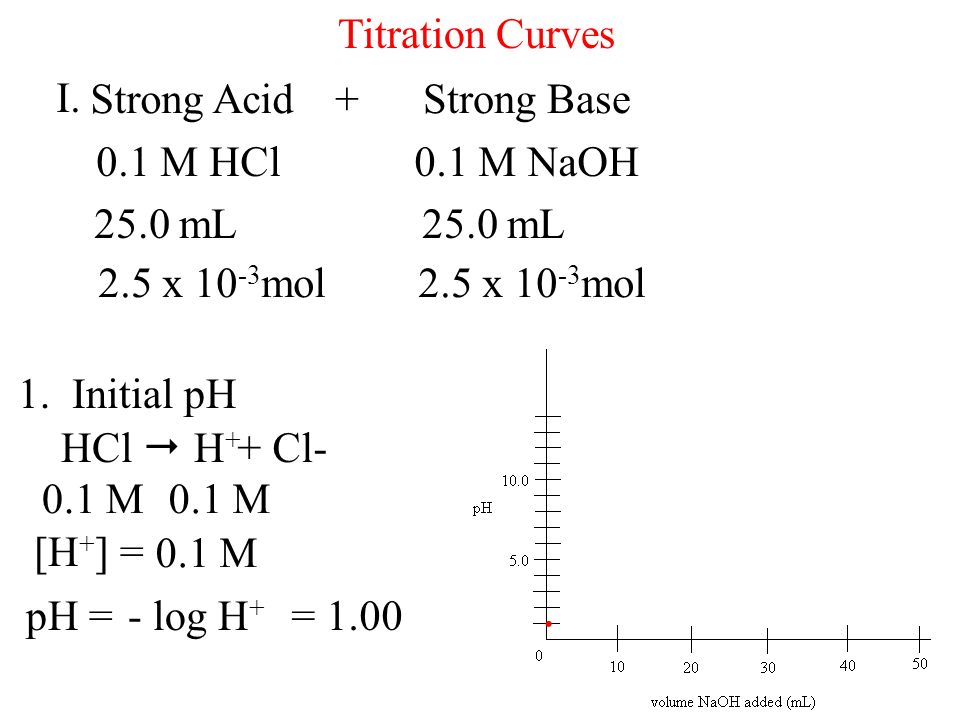
Calculate the pH of a solution obtained by mixing of 100 ml of 0.1 M HCl and 100 ml of 0.2 M - CBSE Class 11 Chemistry - Learn CBSE Forum

Calculate the ph of a solution formed by mixing 100 ml of 0.1m hcl and 9.9ml of 1m naoh - Brainly.in
Calculate the pH of solution obtained by mixing 10 mL of 0.1 M HCl and 40 ml of 0.2 M H2SO4. - Sarthaks eConnect | Largest Online Education Community
SOLVED: Compare the pH of 0.1 M HCl with the pH of 1.0 M CH3COOH. At equilibrium the concentration of CH3COO– is 0.00424 M. Possible answers: The pH of the HCl solution

Calculate the pH of solution obtained by mixing 10 ml of 0.1M HCl and 40ml of 0.2 M H2SO4 - Brainly.in

SOLVED: 1. Calculate the pH if you added 3 mL of 0.1 M HCL to a) 97 mL of pure water at pH 7, and b) 100 mL of phosphate buffer (0.063
![The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ]. The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].](https://dwes9vv9u0550.cloudfront.net/images/4298277/0914b99c-8837-49a9-86f7-3cbcdb1ec4a6.jpg)
The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].
pH of 0.1 M CH3COOH solution is 3 at 25 degree celcius . if limiting molar conductivity of CH3COO and H+ are 40 and 350 S cm2 mol . the molar conductance at 25 degree for 0.1M CH3COOH
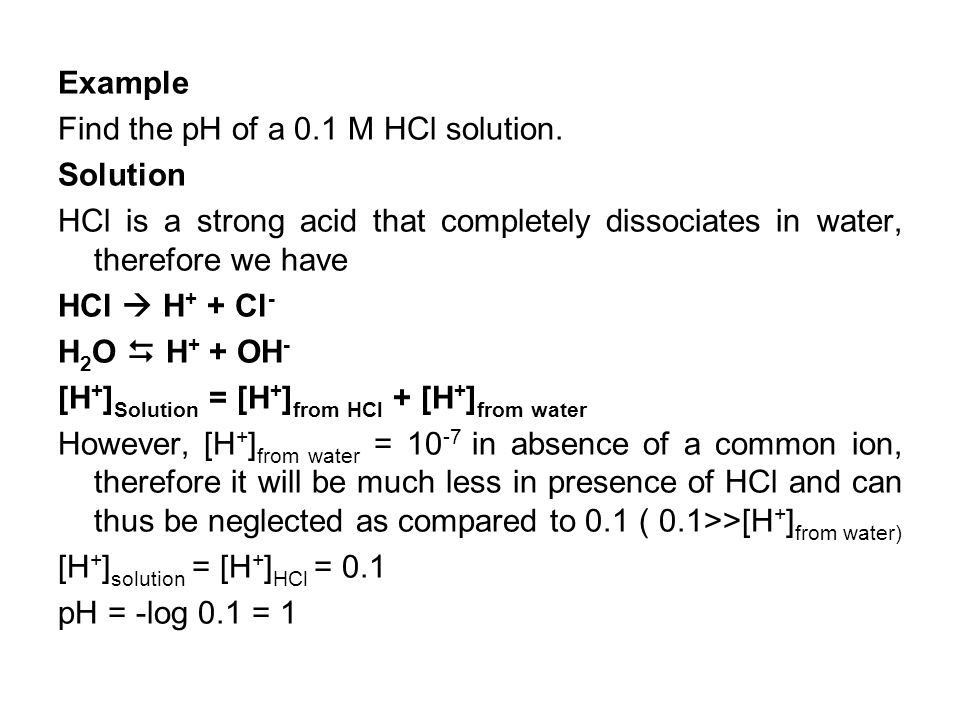




![Calculate pH of following solutions: 0.1 M H2SO4 ( 50 ml ) + 0.4 M HCl 50 (ml) [ log0.3 = - 0.522 ] Calculate pH of following solutions: 0.1 M H2SO4 ( 50 ml ) + 0.4 M HCl 50 (ml) [ log0.3 = - 0.522 ]](https://haygot.s3.amazonaws.com/questions/1330885_1119117_ans_1c33f701c8ed4dc2aa623a201148932c.JPG)