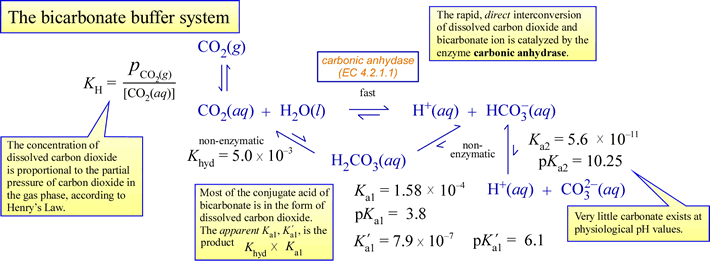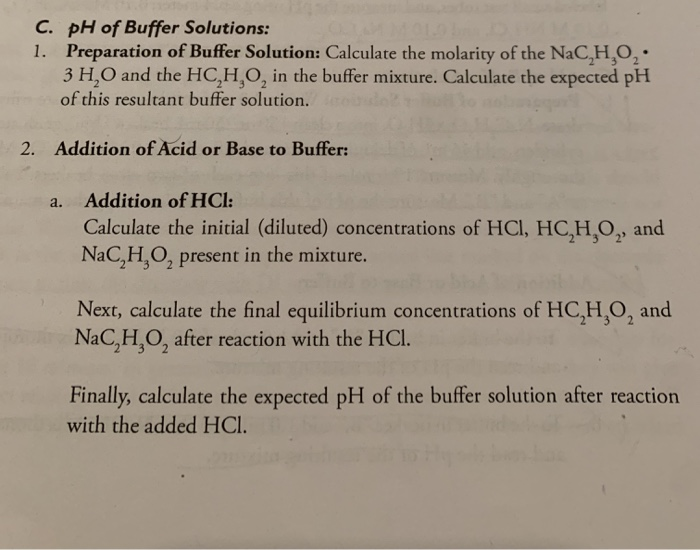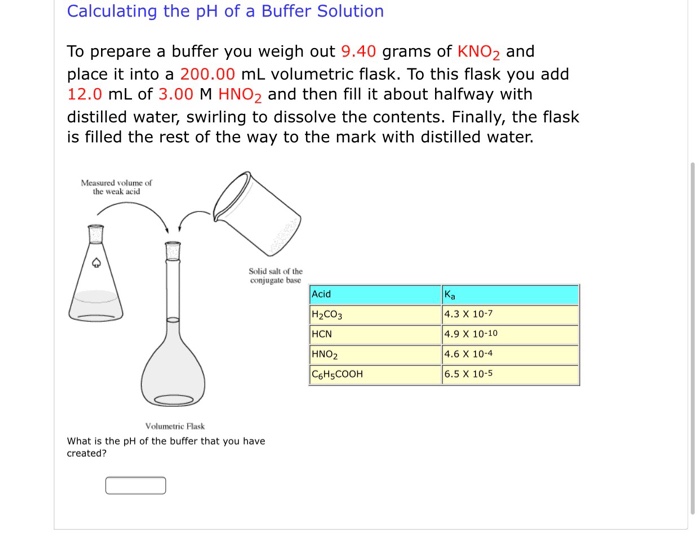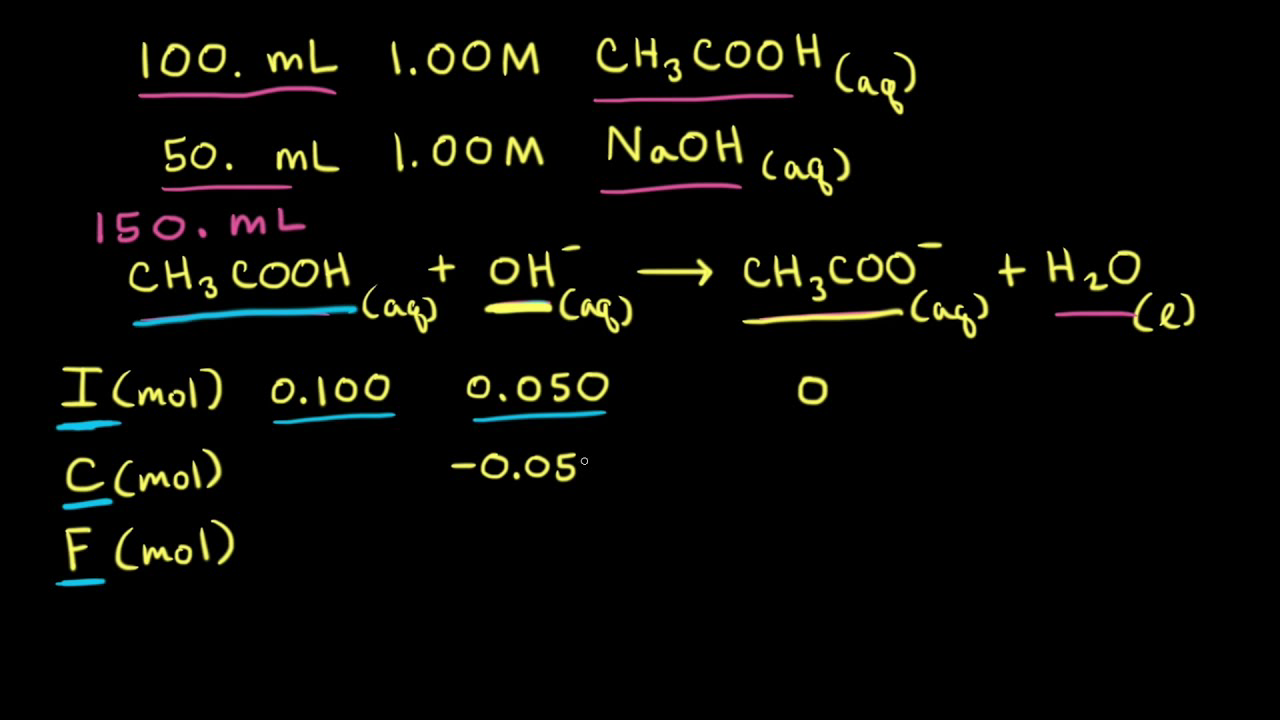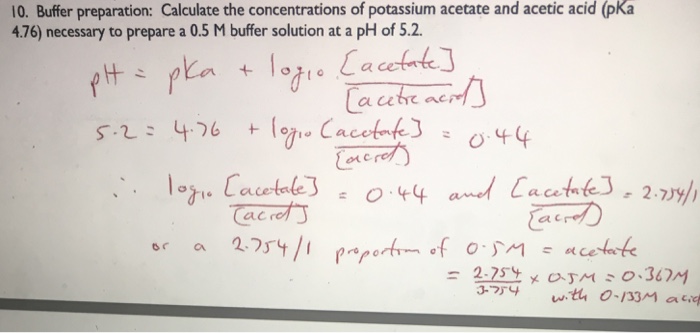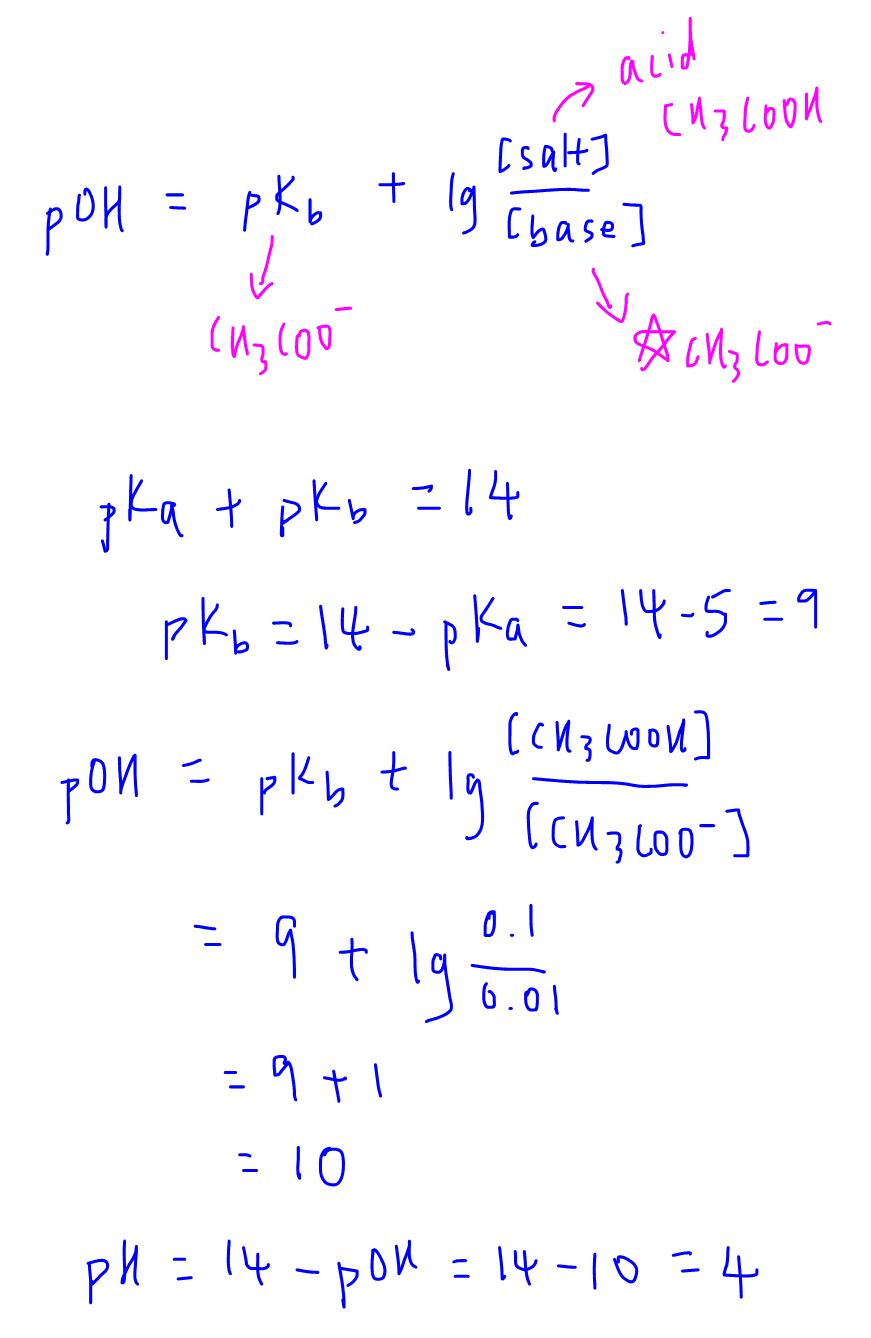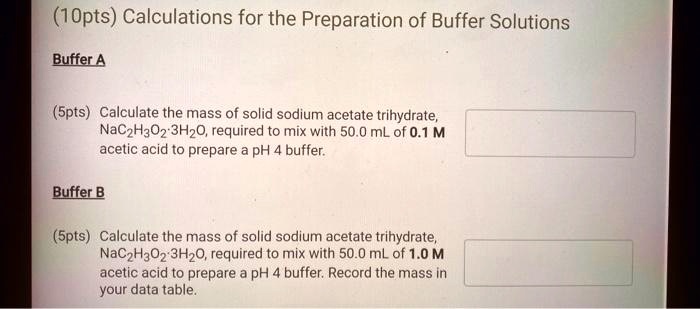
SOLVED: (1Opts) Calculations for the Preparation of Buffer Solutions Buffer A (Spts) Calculate the mass of solid sodium acetate trihydrate, NaCzH302*3H20, required to mix with 50.0 mL of 0.1M acetic acid t0
![SOLVED: (a) basic buffer solution with pH 10.5 is prepared by dissolving NHACl into 30 mL 0.15 M ammonia, ammonium chloride, NH; solution. [Given: Kb for NH; 1.8 X 10-] Calculate the SOLVED: (a) basic buffer solution with pH 10.5 is prepared by dissolving NHACl into 30 mL 0.15 M ammonia, ammonium chloride, NH; solution. [Given: Kb for NH; 1.8 X 10-] Calculate the](https://cdn.numerade.com/ask_images/5b0b09243de64cee9ee5f6101c584fab.jpg)
SOLVED: (a) basic buffer solution with pH 10.5 is prepared by dissolving NHACl into 30 mL 0.15 M ammonia, ammonium chloride, NH; solution. [Given: Kb for NH; 1.8 X 10-] Calculate the
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)
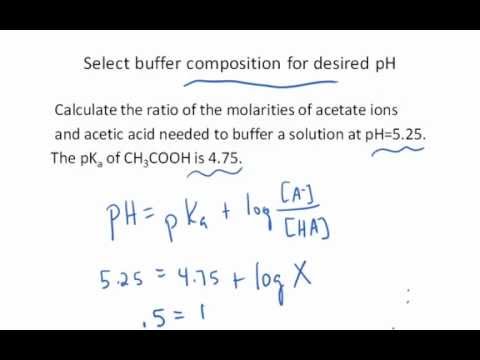
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]