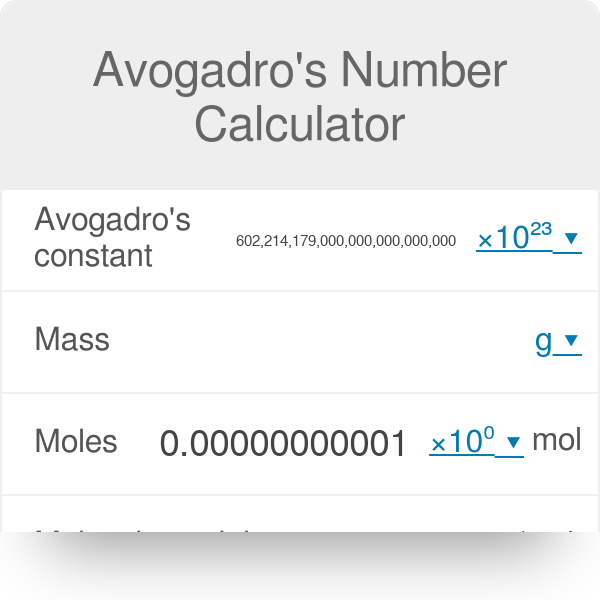
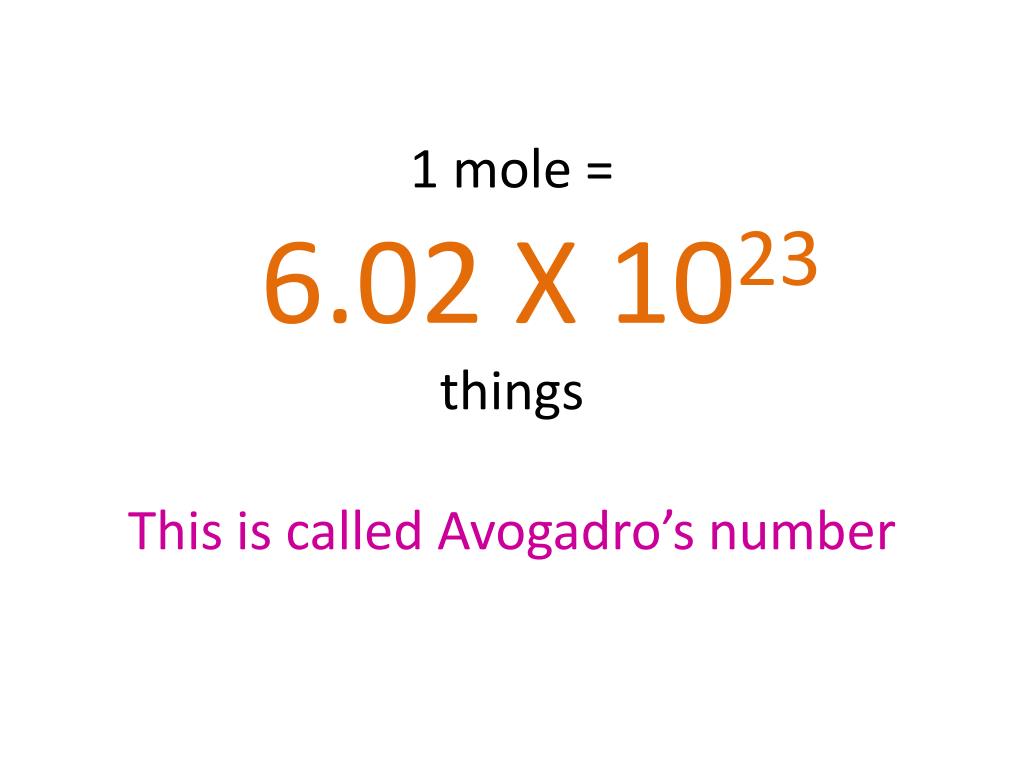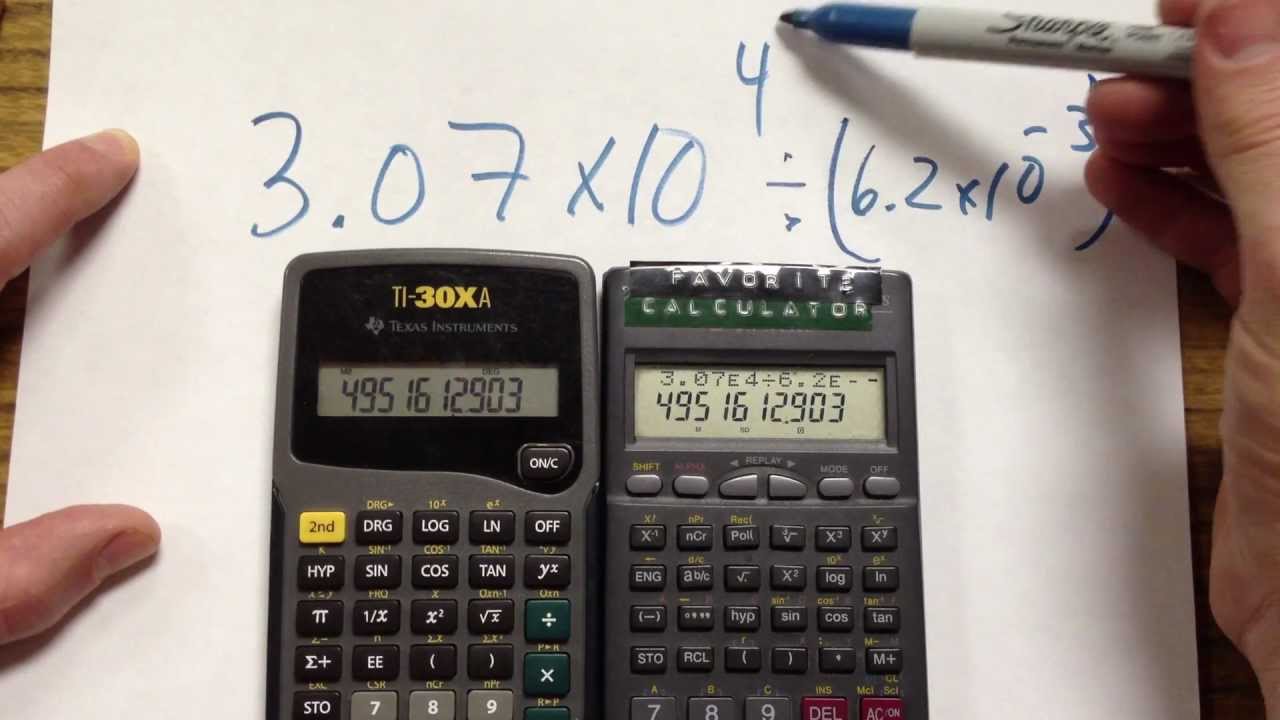I need help with my calculator why is it showing this answer i dont know what i did wrong tho Im using a casio fx-991cw : r/calculators
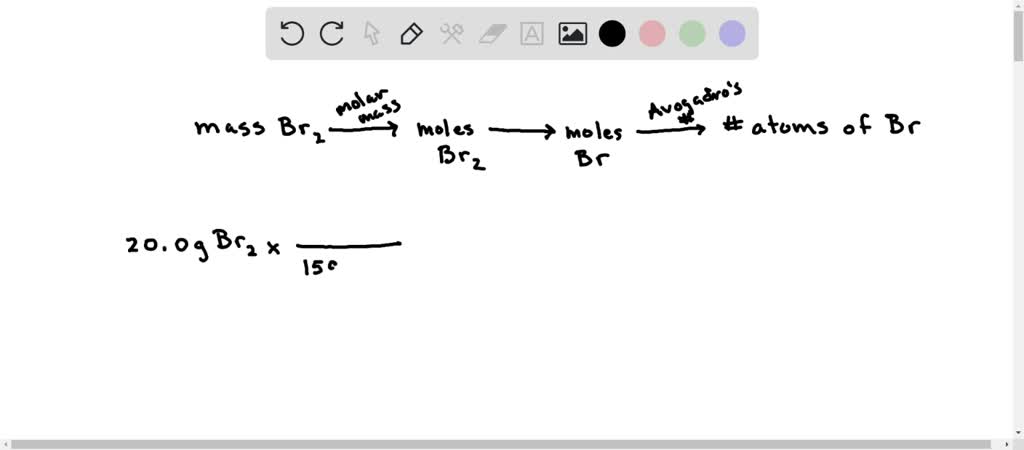
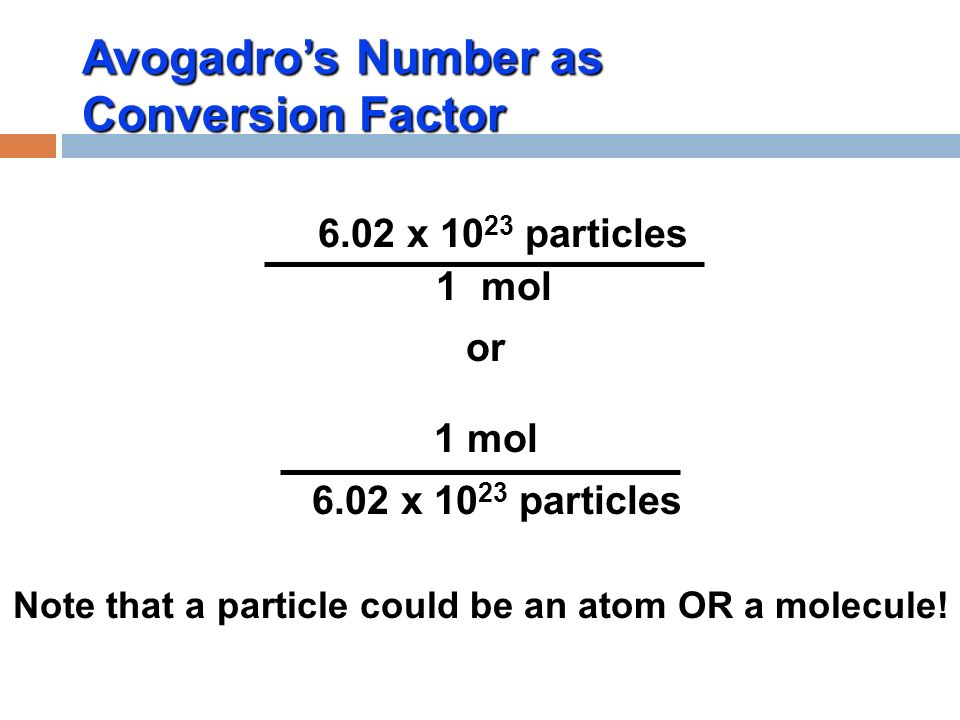
SOLVED: Calculate the number of Br atoms in 20.0g of liquid bromine (Br2). NOTE: Avogadro's number is 6.02 x 1023 A 7.53*10^23 B. 1.51*10^23 C. 3.01*10 ^23 D. 3.77*10^23 E. Nor correct response
Clip of M1.3: The Mole Part 1 (Avogadro's Number) - SchoolTube - Safe video sharing and management for K12

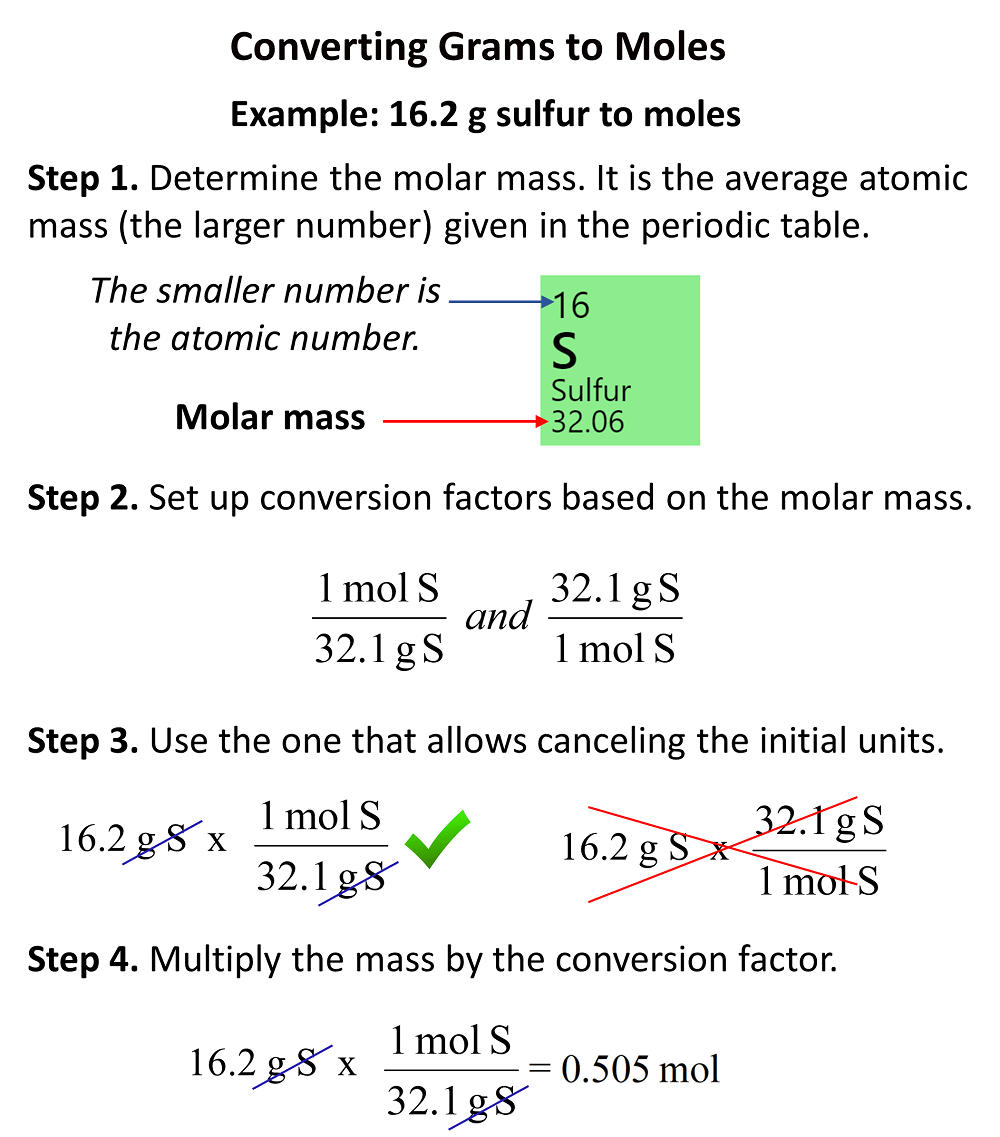
12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)